| CAT NO. |
GBR-3415 |
|---|
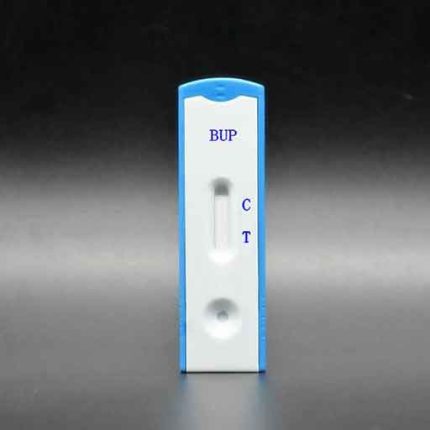
(BUP) Buprenorphine Test (Strip) GBR-3415
ALLTEST Buprenorphine BUP urine drug testing strips
Key points about Buprenorphine BUP drug test strips:
- 10ng sensitivity.
- Detects Buprenorphine (also known as Subutex) for up to 5 days after last use
- 99.8% accuracy
- Quick and easy to use urine drug test strip
- Read result in 3-5 minutes
- Manufactured by ALLTEST
- Full CE & international quality certified test.
- Each drug test kit is an individually foil wrapped urine drug test strip.
Related products
(BUP) Buprenorphine Test (Cassette) GBR-3414
(AMP) Amphetamine Test (Strip) GBR-3409
-
-
- Remove the strip from it's packaging.
- Immerse the strip into the urine with the arrow end pointing towards the urine. DO NOT cover the urine over the MAX (maximum) line.
- Take the strip out of the urine after approximately 15 seconds, and lay it on a flat, non-absorptive clean surface.
- Read results after waiting 5 minutes.
-
-
-
- For medical and other professional in vitro diagnostic use only.
- Do not use after expiration date.
- The test device should remain in the sealed pouch until use.
- The test is for single use only. Do not reuse.
- Wearing gloves is recommended.
-
-
-
- Store as packaged in the sealed pouch at anywhere between 2-30° (36-46°F).
- The test is stable through to the expiration date printed on the sealed pouch.
- The test must remain in the pouch until use.
- Do not freeze.
- Do not use beyond the expiration date.
-
-
-
- Positive: One colour line appears in the control region. No line appears in the test region.
- Negative: Two lines appear; one colour line in the control region, and another apparent colour line in the test region.
- Invalid: Control line fails to appear.
-
Gluten Test (Cassette) GBR-3111
Casein Test (Cassette) GBR-3110
Casein Test Kit
Casein refers to a family of proteins commonly found in mammalian milk. Approximately 80% of bovine milk proteins are caseins which are composed of α-, β- and κ-caseins. This protein fraction is the main component in cheese and is frequently used as a food additive.
Caseins also represent heat-stable allergens. Bovine milk is one of the most important allergenic food ingredients, especially for children. Consequently, the labeling of casein or milk is mandatory in many countries all over the world.
Although there are no legal threshold limits for casein, it is highly recommended that food manufacturers test for very low casein concentrations in order to protect allergic individuals and to avoid allergen-related recalls.
Fast and highly sensitive casein analysis
The Romer Labs casein test kit portfolio covers ELISAs and LFDs for the quantitative and qualitative analysis of casein in environmental samples, rinse waters and finished food products.
Detection of fining agents in wine
Casein is an organic compound frequently used as a fining agent in winemaking. In the fining process, casein is added to bind to suspended particles that will precipitate from the wine.
For wine samples, Romer Labs offers the validated AgraQuant® Casein ELISA and the AgraStrip® Casein LFD with a special wine extraction buffer specifically developed for wine analysis.
Casein Test (Cassette) GBR-3110
Casein Test Kit
Casein refers to a family of proteins commonly found in mammalian milk. Approximately 80% of bovine milk proteins are caseins which are composed of α-, β- and κ-caseins. This protein fraction is the main component in cheese and is frequently used as a food additive.
Caseins also represent heat-stable allergens. Bovine milk is one of the most important allergenic food ingredients, especially for children. Consequently, the labeling of casein or milk is mandatory in many countries all over the world.
Although there are no legal threshold limits for casein, it is highly recommended that food manufacturers test for very low casein concentrations in order to protect allergic individuals and to avoid allergen-related recalls.
Fast and highly sensitive casein analysis
The Romer Labs casein test kit portfolio covers ELISAs and LFDs for the quantitative and qualitative analysis of casein in environmental samples, rinse waters and finished food products.
Detection of fining agents in wine
Casein is an organic compound frequently used as a fining agent in winemaking. In the fining process, casein is added to bind to suspended particles that will precipitate from the wine.
For wine samples, Romer Labs offers the validated AgraQuant® Casein ELISA and the AgraStrip® Casein LFD with a special wine extraction buffer specifically developed for wine analysis.






Reviews
There are no reviews yet.