Inside the USA: for research use only(RUO) or LDT
(AMP) Amphetamine Test (Cassette) GBR-3408
Directions for use:
-
-
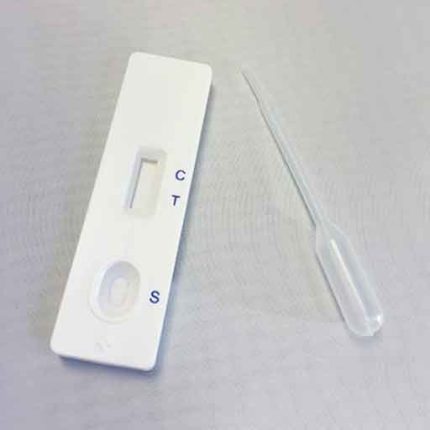
- Remove the test device from its foil wrapper by tearing along the slice.
- Using the specimen dropper, withdraw urine sample from the specimen cup and slowly dispense three drops (approx 120 UI) into the circular sample well.
- Be careful not to overfill the absorbent pad.
- Read results after 5 minutes.
-
-
-
- For medical and other professional in vitro diagnostic use only.
- Do not use after expiration date.
- The test device should remain in the sealed pouch until use.
- The test is for single use only. Do not reuse.
- Wearing gloves is recommended.
-
-
-
- Store as packaged in the sealed pouch at anywhere between 2-30° (36-46°F).
- The test is stable through to the expiration date printed on the sealed pouch.
- The test must remain in the pouch until use.
- Do not freeze.
- Do not use beyond the expiration date.
-
-
- Positive: One colour line appears in the control region. No line appears in the test region.
- Negative: Two lines appear; one colour line in the control region, and another apparent colour line in the test region.
- Invalid: Control line fails to appear.
(AMP) Amphetamine Test (Cassette) GBR-3408
Directions for use:
-
-
- Remove the test device from its foil wrapper by tearing along the slice.
- Using the specimen dropper, withdraw urine sample from the specimen cup and slowly dispense three drops (approx 120 UI) into the circular sample well.
- Be careful not to overfill the absorbent pad.
- Read results after 5 minutes.
-
-
-
- For medical and other professional in vitro diagnostic use only.
- Do not use after expiration date.
- The test device should remain in the sealed pouch until use.
- The test is for single use only. Do not reuse.
- Wearing gloves is recommended.
-
-
-
- Store as packaged in the sealed pouch at anywhere between 2-30° (36-46°F).
- The test is stable through to the expiration date printed on the sealed pouch.
- The test must remain in the pouch until use.
- Do not freeze.
- Do not use beyond the expiration date.
-
-
- Positive: One colour line appears in the control region. No line appears in the test region.
- Negative: Two lines appear; one colour line in the control region, and another apparent colour line in the test region.
- Invalid: Control line fails to appear.
(AMP) Amphetamine Test (Strip) GBR-3409
Directions for use:
-
-
- Remove the strip from it's packaging.
- Immerse the strip into the urine with the arrow end pointing towards the urine. DO NOT cover the urine over the MAX (maximum) line.
- Take the strip out of the urine after approximately 15 seconds, and lay it on a flat, non-absorptive clean surface.
- Read results after waiting 5 minutes.
-
-
-
- For medical and other professional in vitro diagnostic use only.
- Do not use after expiration date.
- The test device should remain in the sealed pouch until use.
- The test is for single use only. Do not reuse.
- Wearing gloves is recommended.
-
-
-
- Store as packaged in the sealed pouch at anywhere between 2-30° (36-46°F).
- The test is stable through to the expiration date printed on the sealed pouch.
- The test must remain in the pouch until use.
- Do not freeze.
- Do not use beyond the expiration date.
-
-
-
- Positive: One colour line appears in the control region. No line appears in the test region.
- Negative: Two lines appear; one colour line in the control region, and another apparent colour line in the test region.
- Invalid: Control line fails to appear.
-
(AMP) Amphetamine Test (Strip) GBR-3409
Directions for use:
-
-
- Remove the strip from it's packaging.
- Immerse the strip into the urine with the arrow end pointing towards the urine. DO NOT cover the urine over the MAX (maximum) line.
- Take the strip out of the urine after approximately 15 seconds, and lay it on a flat, non-absorptive clean surface.
- Read results after waiting 5 minutes.
-
-
-
- For medical and other professional in vitro diagnostic use only.
- Do not use after expiration date.
- The test device should remain in the sealed pouch until use.
- The test is for single use only. Do not reuse.
- Wearing gloves is recommended.
-
-
-
- Store as packaged in the sealed pouch at anywhere between 2-30° (36-46°F).
- The test is stable through to the expiration date printed on the sealed pouch.
- The test must remain in the pouch until use.
- Do not freeze.
- Do not use beyond the expiration date.
-
-
-
- Positive: One colour line appears in the control region. No line appears in the test region.
- Negative: Two lines appear; one colour line in the control region, and another apparent colour line in the test region.
- Invalid: Control line fails to appear.
-
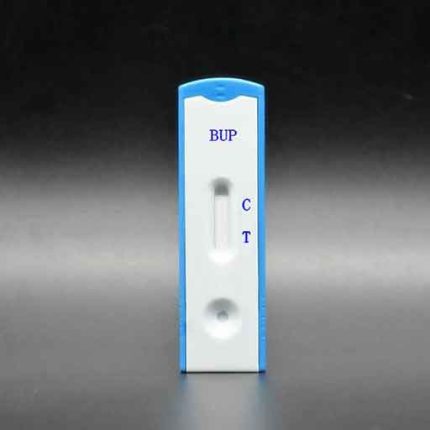
(BUP) Buprenorphine Test (Cassette) GBR-3414
sold under the trade names Subutex,Buprenex,Temgesic and Suboxone, which contain Buprenorphine HCl alone or in combination with Naloxone HCl.Therapeutically, Buprenorphine is used as a substitution treatment for opioid addicts. Substitution treatment is a form of medical care offered to opiate addicts (primarily heroin addicts) based on a similar or identical substance to the drug normally used
1.Application
Buprenorphine (BUP) Single Drug Test Cassette is a rapid urine drug screening test that can be performed without the use of an instrument.
Buprenorphine is a potent analgesic often used in the treatment of opioid addiction.The drug is sold under the trade names Subutex,Buprenex,Temgesic and Suboxone, which contain Buprenorphine HCl alone or in combination with Naloxone HCl.Therapeutically, Buprenorphine is used as a substitution treatment for opioid addicts. Substitution treatment is a form of medical care offered to opiate addicts (primarily heroin addicts) based on a similar or identical substance to the drug normally used.
2.Principle
Buprenorphine (BUP) Drug Test Device is an immunoassay based on the principle of competitive binding.Drugs which may be present in the urine specimen compete against the drug conjugate for binding sites on the antibody.
One Step Buprenorphine Drug Test Device is a rapid urine drug screening test that can be performed without the use of an instrument.During testing,a urine specimen migrates upward by capillary action. Buprenorphine, if present in the urine specimen below 10 ng/mL,will not saturate the binding sites of antibody-coated particles in the test.
The antibody-coated particles will then be captured by immobilized Buprenorphine conjugate and a visible colored line will show up in the test line region.
The colored line will not form in the test line region if the Buprenorphine level exceeds 10 ng/mL because it will saturate all the binding sites of anti-Buprenorphine antibodies.
A drug-positive urine specimen will not generate a colored line in the test line region because of drug competition,while a drug-negative urine specimen or a specimen containing a drug concentration lower than the cut-off will generate a line in the test line region.
To serve as a procedural control,a colored line will always appear at the control line region,indicating that proper volume of specimen has been added and membrane wicking has occurred.
(BUP) Buprenorphine Test (Cassette) GBR-3414
sold under the trade names Subutex,Buprenex,Temgesic and Suboxone, which contain Buprenorphine HCl alone or in combination with Naloxone HCl.Therapeutically, Buprenorphine is used as a substitution treatment for opioid addicts. Substitution treatment is a form of medical care offered to opiate addicts (primarily heroin addicts) based on a similar or identical substance to the drug normally used
1.Application
Buprenorphine (BUP) Single Drug Test Cassette is a rapid urine drug screening test that can be performed without the use of an instrument.
Buprenorphine is a potent analgesic often used in the treatment of opioid addiction.The drug is sold under the trade names Subutex,Buprenex,Temgesic and Suboxone, which contain Buprenorphine HCl alone or in combination with Naloxone HCl.Therapeutically, Buprenorphine is used as a substitution treatment for opioid addicts. Substitution treatment is a form of medical care offered to opiate addicts (primarily heroin addicts) based on a similar or identical substance to the drug normally used.
2.Principle
Buprenorphine (BUP) Drug Test Device is an immunoassay based on the principle of competitive binding.Drugs which may be present in the urine specimen compete against the drug conjugate for binding sites on the antibody.
One Step Buprenorphine Drug Test Device is a rapid urine drug screening test that can be performed without the use of an instrument.During testing,a urine specimen migrates upward by capillary action. Buprenorphine, if present in the urine specimen below 10 ng/mL,will not saturate the binding sites of antibody-coated particles in the test.
The antibody-coated particles will then be captured by immobilized Buprenorphine conjugate and a visible colored line will show up in the test line region.
The colored line will not form in the test line region if the Buprenorphine level exceeds 10 ng/mL because it will saturate all the binding sites of anti-Buprenorphine antibodies.
A drug-positive urine specimen will not generate a colored line in the test line region because of drug competition,while a drug-negative urine specimen or a specimen containing a drug concentration lower than the cut-off will generate a line in the test line region.
To serve as a procedural control,a colored line will always appear at the control line region,indicating that proper volume of specimen has been added and membrane wicking has occurred.
(BUP) Buprenorphine Test (Strip) GBR-3415
ALLTEST Buprenorphine BUP urine drug testing strips
Key points about Buprenorphine BUP drug test strips:- 10ng sensitivity.
- Detects Buprenorphine (also known as Subutex) for up to 5 days after last use
- 99.8% accuracy
- Quick and easy to use urine drug test strip
- Read result in 3-5 minutes
- Manufactured by ALLTEST
- Full CE & international quality certified test.
- Each drug test kit is an individually foil wrapped urine drug test strip.
(BUP) Buprenorphine Test (Strip) GBR-3415
ALLTEST Buprenorphine BUP urine drug testing strips
Key points about Buprenorphine BUP drug test strips:- 10ng sensitivity.
- Detects Buprenorphine (also known as Subutex) for up to 5 days after last use
- 99.8% accuracy
- Quick and easy to use urine drug test strip
- Read result in 3-5 minutes
- Manufactured by ALLTEST
- Full CE & international quality certified test.
- Each drug test kit is an individually foil wrapped urine drug test strip.
(BZO) Benzodiazepine Test (Cassette) GBR-3412
Note:Could be customized under your request
Specifications
1. High sensitivity, simple, easy.
2. Accuracy: >99%.
3. CE & ISO approved.
4. Specimen: Urine.
5. Model No:BZO-U02D
1.Application
The BZO Rapid Test Device (Urine) detects Benzodiazepines through visual interpretation of color development
on the Device. Drug conjugates are immobilized on the test region of the membrane. During testing, the specimen
reacts with antibodies conjugated to colored particles and precoated on the sample pad. The mixture then
migratesthrough the membrane by capillary action, and interacts with reagents on the membrane. If there are
insufficient drug molecules in the specimen, the antibody-colored particle conjugate will bind to the drug conjugates,
forming a colored band at the test region of the membrane. Therefore, a colored band appears in the test region
when the urine is negative for the drug. If drug molecules are present in the urine above the cut-off concentration
of the test, they compete with the immobilized drug conjugate on the test region for limited antibody binding sites.
This will prevent attachment of the antibody-colored particle conjugate to the test region. Therefore, the absence
of a colored band at the test region indicates a positive result. The appearance of a colored band at the control
region serves as a procedural control, indicating that the proper volume of specimen has been added and
membrane wicking has occurred.
2. Usage:
Allow test device,urine specimen,and/or controls to equilibrate to room temperature (15-30°C) prior to testing.